![]() What exactly are biomolecules? Let’s analyze the word. Click on each part of the word to learn more about what biomolecules are.
What exactly are biomolecules? Let’s analyze the word. Click on each part of the word to learn more about what biomolecules are.
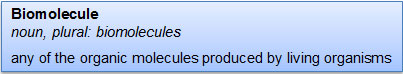
If you look up the definition of biomolecule in the dictionary, you will see a definition similar to the one below.

Source: Organic sign in grocery store, Organic Authority
This definition includes another important term: organic molecules. You have probably heard the word organic. Have you ever noticed the organic produce section in the grocery store? When you see the term organic in the grocery store or on food labels it typically means the food items were grown, processed, and packaged using no man-made fertilizers or pesticides.
In chemistry, organic means something different. Look at the definition of organic molecule below.
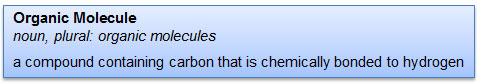
Many people mistakenly define organic molecules as compounds containing carbon. But if you look carefully at the definition, you see that in organic molecules, carbon is covalently bonded to hydrogen. There can be other atoms present in organic compounds as well, but there must be at least one carbon bonded to hydrogen. Organic molecules are often referred to as hydrocarbons because of the carbon and hydrogen bond.
![]() Look at the structures below. Which ones are organic molecules? Click on the ones that are organic molecules.
Look at the structures below. Which ones are organic molecules? Click on the ones that are organic molecules.
So, what is so special about carbon? Pictured below is the periodic table entry for carbon.
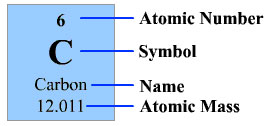
Source: Periodic table entry of carbon, Thinkquest
From the periodic table, you can tell that carbon has six protons, six neutrons, and six electrons. The image below shows a Bohr model drawing of carbon.
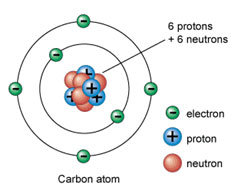
Source: Carbon Atom, Universe Today
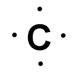
Carbon has four valence electrons. Remember, it is the valence electrons, electrons on the outer energy level, which are involved in bonding. The image to the right shows the Lewis Dot structure of carbon.
These four valence electrons allow carbon to form strong covalent bonds with other elements including hydrogen, oxygen, phosphorus, sulfur, and nitrogen.
Carbon atoms can also bond with other carbon atoms. Numerous carbon atoms can bond to make long straight, branched chains or ring-shaped structures. The pictures below shows the variety of shapes carbon-to-carbon bonds can form.
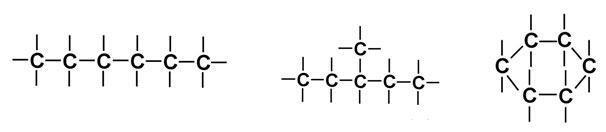
Carbon-to-carbon bonds can be in the form of single, double and triple bonds as shown in the diagrams below.
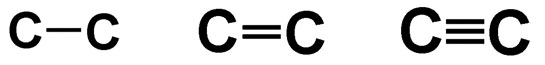
Now, that you can properly identify organic molecules, and you know a little more about carbon, let’s go back to the term biomolecule.
There are four main classes of biomolecules: carbohydrates, lipids, proteins, and nucleic acids.

All organic compounds have a single basic building unit called a monomer. Monomer comes from the Greek words monos, meaning “single” and meros meaning “part.” Monomers join together to form polymers. The prefix poly comes from the Greek word polus, meaning “many," so polymer means “many parts.” The process of dehydration synthesis or dehydration condensation forms Biomolecular polymers.
The elements carbon, hydrogen, oxygen, phosphorus, sulfur, and nitrogen, in different combinations, make up each of the molecules. How these elements are arranged dictates the type of molecule that’s formed.