
Source: French Fries, Road Food

Source: French Fries, Road Food
Have you ever eaten a batch of french fries and noticed spots on the paper under your fries? Those spots are from the oil that the fries were cooked in. Fats, oils, steroids, and waxes make up the group of biomolecules known as lipids.
Lipids are large biomolecules that are insoluble in water, meaning they do not dissolve in water. Lipids are made of carbon, hydrogen, and oxygen atoms and can easily be identified by the large number of carbon and hydrogen atoms.
There are three main classes of lipids: triglycerides, phospholipids, and steroids.
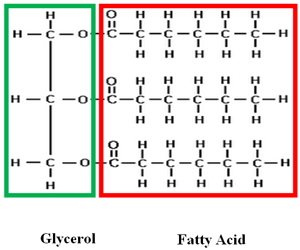
Triglycerides are probably what you think most of when you hear the word lipids. Triglycerides include fats and oils. If you take a closer look at the word triglyceride, you notice it has the prefix tri–. Tri means three. Lipids are made up of two main parts: a glycerol molecule with three fatty acids attached.
Fatty acids are long chains of carbon (usually 14-24 carbons) and hydrogen. Potential energy is stored in these carbon-hydrogen bonds. In triglycerides, there are a lot of carbon- hydrogen bonds. Organisms convert excess energy to fat. The main function of triglycerides is long-term energy storage. Fat also helps insulate, or keep animals warm, as well as protects organs.
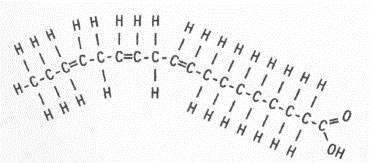
![]() There are two types of fatty acids: saturated and unsaturated. The images below show a saturated fatty acid and an unsaturated fatty acid. Can you see the difference in the two structures? Click on the image to see the difference.
There are two types of fatty acids: saturated and unsaturated. The images below show a saturated fatty acid and an unsaturated fatty acid. Can you see the difference in the two structures? Click on the image to see the difference.
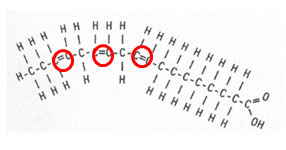
Notice in the unsaturated fatty acid there is a double bond between two of the carbons. This double bond is known as the point of unsaturation. Each point of unsaturation reduces the number of hydrogen atoms present in the compound by two. The picture below shows a polyunsaturated fatty acid. In a polyunsaturated acid, there is more than one double bond.
How many hydrogen atoms would reduce this molecule?


Source: Western Pack Butter, Steve Karg, Wikimedia Commons
Saturated fats are found in animals. Most saturated fats have a high melting point so that it can return to a solid state at room temperature. Butter is an example of a saturated triglyceride.

Source: Crisco Vegetable Oil, Wal-Mart
Unsaturated fats are found in plants. Unsaturated fats have a lower melting point and are in liquid form at room temperature. Cooking oils such as vegetable oil, peanut oil, olive oil, and canola oil are unsaturated triglycerides.

Source: Crisco Vegetable Oil, Wal-Mart
What about products such as Crisco shorting; is it a saturated or unsaturated triglyceride? Notice the label clearly says “all-vegetable” yet shorting is solid. How is this possible?

Source: Crisco Ingredients, Fitness Agon
Products such as shorting are hydrogenated. During the hydrogenation process, hydrogen atoms are added. If you look at the ingredients list on products such as shorting, you will notice it says “fully hydrogenated” or “partially hydrogenated.”
When you were studying the structure of the cell you probably heard the phrase “phospholipid bilayer” in reference to the cell membrane. Phospholipids create the structure of cell membranes.
Phospholipids have a similar structure to triglycerides except phospholipids have a glycerol, two fatty acids, and a phosphate molecule.
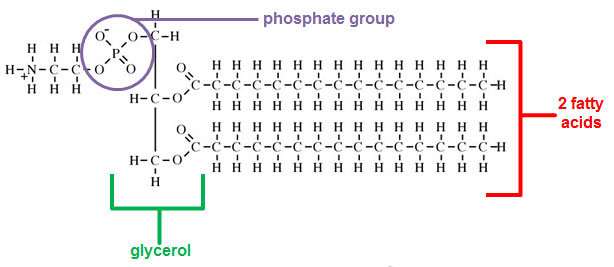
Source: lecithin, Indiana University
Look below at the illustration of the cell membrane.
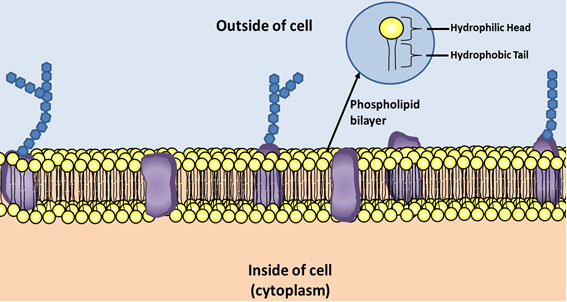
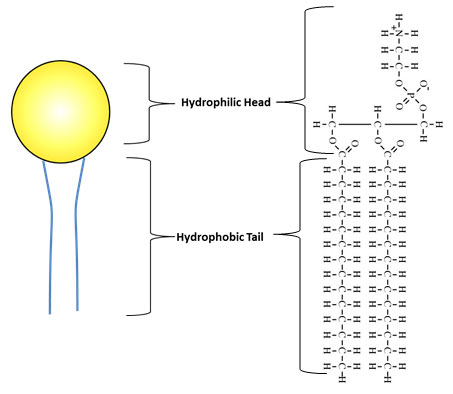
The hydrophilic (water loving) head of the phospholipid is the glycerol and the phosphate group. The hydrophobic (water fearing) tail is a fatty acid. Hydrophobic molecules are repelled by water.
The images below compare the illustration of a phospholipid to the structural formula.

When you hear the word steroids, you probably think of something that is bad for you or news stories of steroid abuse by professional athletes. But, not all steroids are bad for you! In fact, you have steroids pumping through your body right now that are essential to your health and proper body function. Steroids are lipids that act as hormones. Hormones function as chemical messengers for the body. There are hundreds of different steroids found in fungi, plants, and animals.
Steroids, like triglycerides and phospholipids are hydrophobic but the structure of steroids is completely different than the structure of the other classes of lipids. The basic structure of all steroids is four carbon rings bonded together. The basic structure of a steroid is shown in the image below.
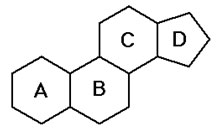
Source: Steroid Skeleton, Portland State University
![]() What make each steroid different from other steroids are the chemical groups attached to the four carbon rings. Two common steroids are shown below. Click on each image to learn about the function of that particular steroid.
What make each steroid different from other steroids are the chemical groups attached to the four carbon rings. Two common steroids are shown below. Click on each image to learn about the function of that particular steroid.
3. What are the main classes of lipids?
4. What are the three primary functions of lipids?