
Inside your body there are thousands of different chemical reactions happening each minute! The elements or compounds that enter a reaction are called the reactants, and the elements or compounds produced by the chemical reaction are called products.
Energy is released or absorbed whenever chemical reactions take place due to the breaking or forming of chemical bonds. Chemical reactions that release energy often, but not always, occur on their own while chemical reactions that absorb energy will not occur without a source of energy such as ATP. The energy needed to get a reactions started is called activation energy.
| Think of this analogy. If you were going to bake cupcakes and you put all the ingredients (reactants) together, you would not simply make the batter and wait around for it to turn into cupcakes. You need to place the batter in the oven so enough energy goes into it to make it into cupcakes- the product. |  |
![]() Watch this short video about the importance of enzymes.
Watch this short video about the importance of enzymes.
Enzymes are proteins that act as catalysts. Catalysts increase the rates of chemical reactions. Enzymes catalyze a reaction by lowering the activation energy; which has a dramatic effect on how quickly the reaction is completed. Enzyme-catalyzed reactions occur extremely fast. They happen about a million times faster than uncatalyzed reactions.
Look at the graph below. It shows the effects of enzymes on a reaction.
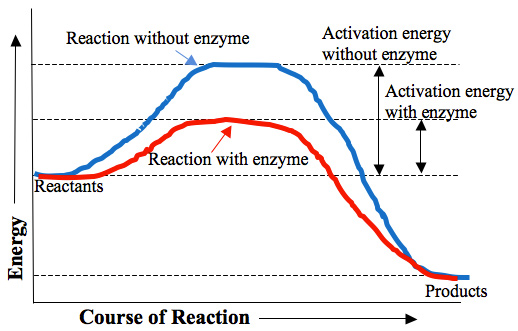
The blue line on the graph shows the reaction taking place without the enzyme and the red line shows the reaction with the enzyme. Notice that the activation energy needed for the reaction with the enzyme is much lower than the reaction without the enzyme.